Antleron and Novasign to join forces in ATMP Modeling
The Potential
Advanced therapy medicinal products (ATMPs) are targeted therapies based on genes, cells, or tissues that deliver a therapeutic benefit to a patient-specific population and often treat rare diseases or improve upon existing treatments. The global ATMP market is expected to reach approximately 21.2 billion dollars by 2028. With an expected compound annual growth rate of 13.2%, the market surpasses the total biopharmaceutical market by a factor of three. More and more companies grasp the potential value of ATMPs, and we are seeing new products coming to the market at an increasing pace.
Limited understanding
As ATMPs consist of a diverse group of relatively novel products, sustainable manufacturing requires innovative development routes, and the ATMP market access has not matured yet as shown by the commercial hurdles the first generation of ATMPs encountered. With these novel types of products, new strategies have to be defined to describe, develop, and sustainably implement the production process in the best possible way. Key to the creation of an ATMP process toolbox are modeling innovations that enable efficient and effective experimentation, in order to spend the available resources as efficiently as possible. Due to the challenges of inherent biological complexity, unrivaled raw material variability, and immature production technologies, more advanced production approaches have to be identified and characterized in the best possible way to yield higher process understanding and to increase process robustness and accelerate process development.
Joint Forces

Antleron and Novasign announce their collaboration in developing advanced process modeling strategies for ATMP manufacturing to understand, describe and predict personalized or customized bioreactor-based adherent cell manufacturing processes in a better and more reliable way. Antleron’s strong background in the co-development of improved and scalable bioprocess solutions is building one of the foundations to success for custom cell manufacturing routes. This is achieved through merging the potential of cells, additive manufacturing, bioreactors with computational and experimental process engineering tools.
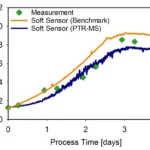
Our hybrid modeling approach on the other hand is the perfect fit to describe cell growth and product formation in the most reliable way and with a minimum set of experiments. This approach offers to grow the collaborative toolbox and enable scalable solutions. One of the targeted solutions is model-based process optimization (we will release an article about this soon), in which process optima can be found faster and with a reduced experimental load. In the next step, the models will be used for monitoring and control of the optimized process as already described in a project with Siemens. As a first focus, we are exploring the added value of merging methodologies and technologies such as risk-based process development, model-based Design of Experiments, soft-sensing, and hybrid modeling in combination with Computational Fluid Dynamics (CFD) to optimize cell expansion of adherent cells in a bioreactor.
We are looking forward to delivering the first solutions together soon! Stay tuned!