Viral Vector Manufacturing: 64% Fewer Experiments in Lytic Virus Production
Hybrid Modeling for Efficient Process Development
In a recent project with our strategic partner bespark*bio, we combined our hybrid modeling with best-in-class bioprocessing expertise to maximize the efficiency of a Vero cell culture process producing a lytic virus. This approach to process optimization ensured that instead of requiring 22 experimental runs, the model effectively characterized the experimental space with just 8 strategically selected experiments. This reduction represents a 64% decrease in experimental effort, leading to substantial savings in time, resources, and operational costs.
Simulating a 3D Experimental Design
In this showcase, our model enabled a detailed simulation of the three-dimensional experimental design. The 8 selected runs were spaced far apart across the experimental space in two experimental blocks involving the following key process parameters:
- MOI (Multiplicity of Infection)
- Temperature
- pH
We then systematically changed the above process parameters to investigate their effect on:
- Process Performance
- Viable cell count (VCC)
- Virus titer
- Product Quality
- Double-stranded DNA (dsDNA)
- Host cell proteins (HCP)
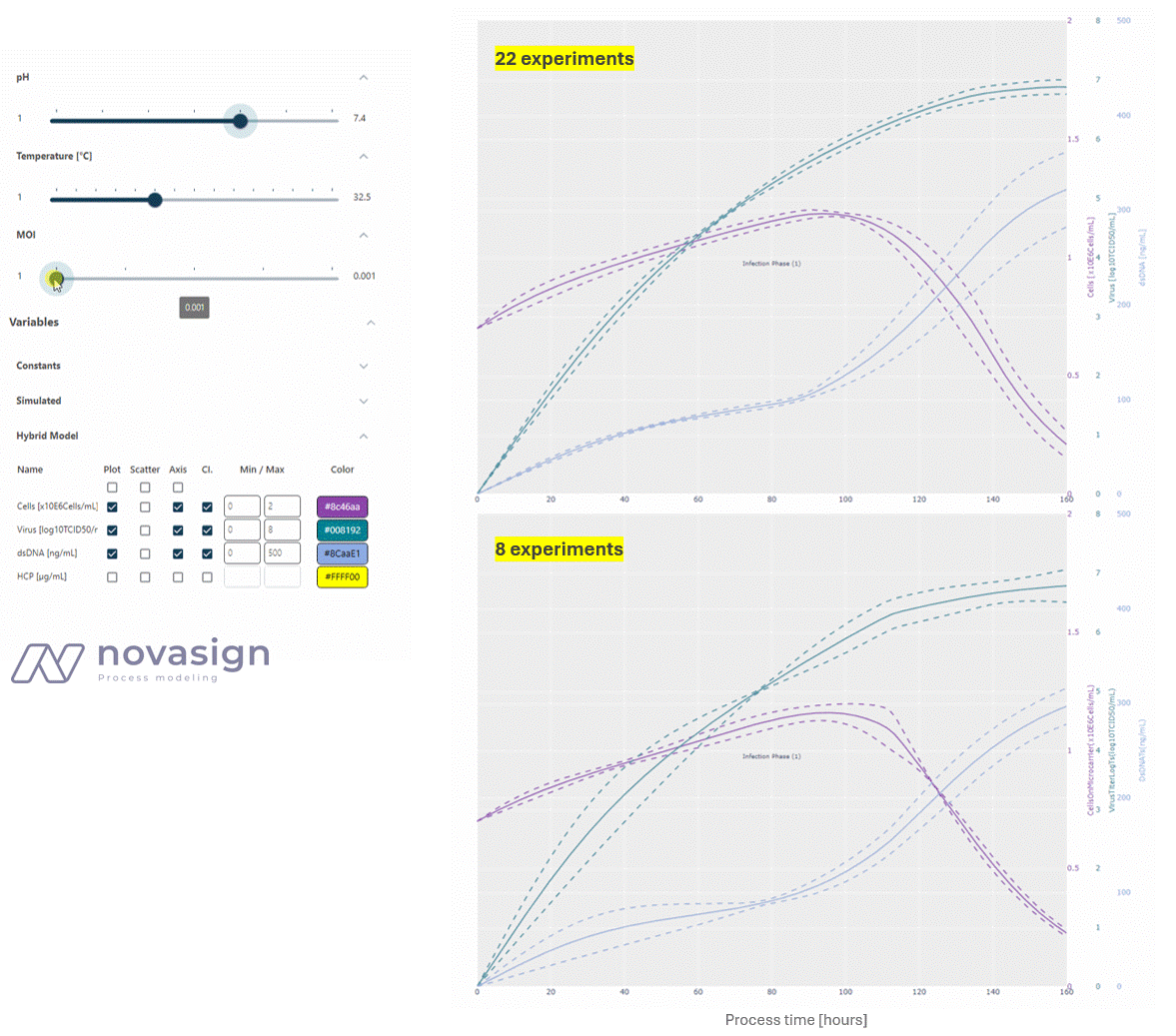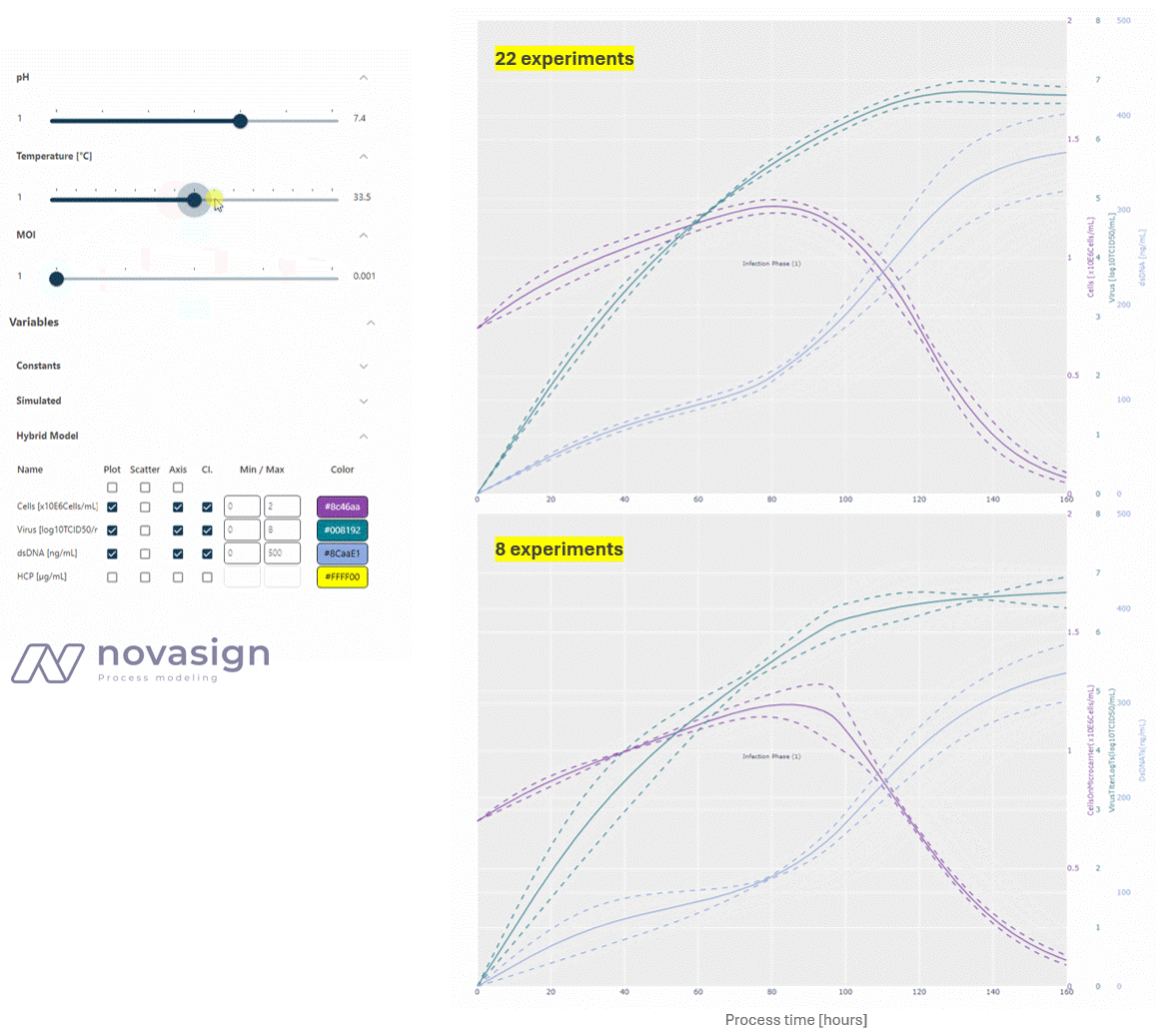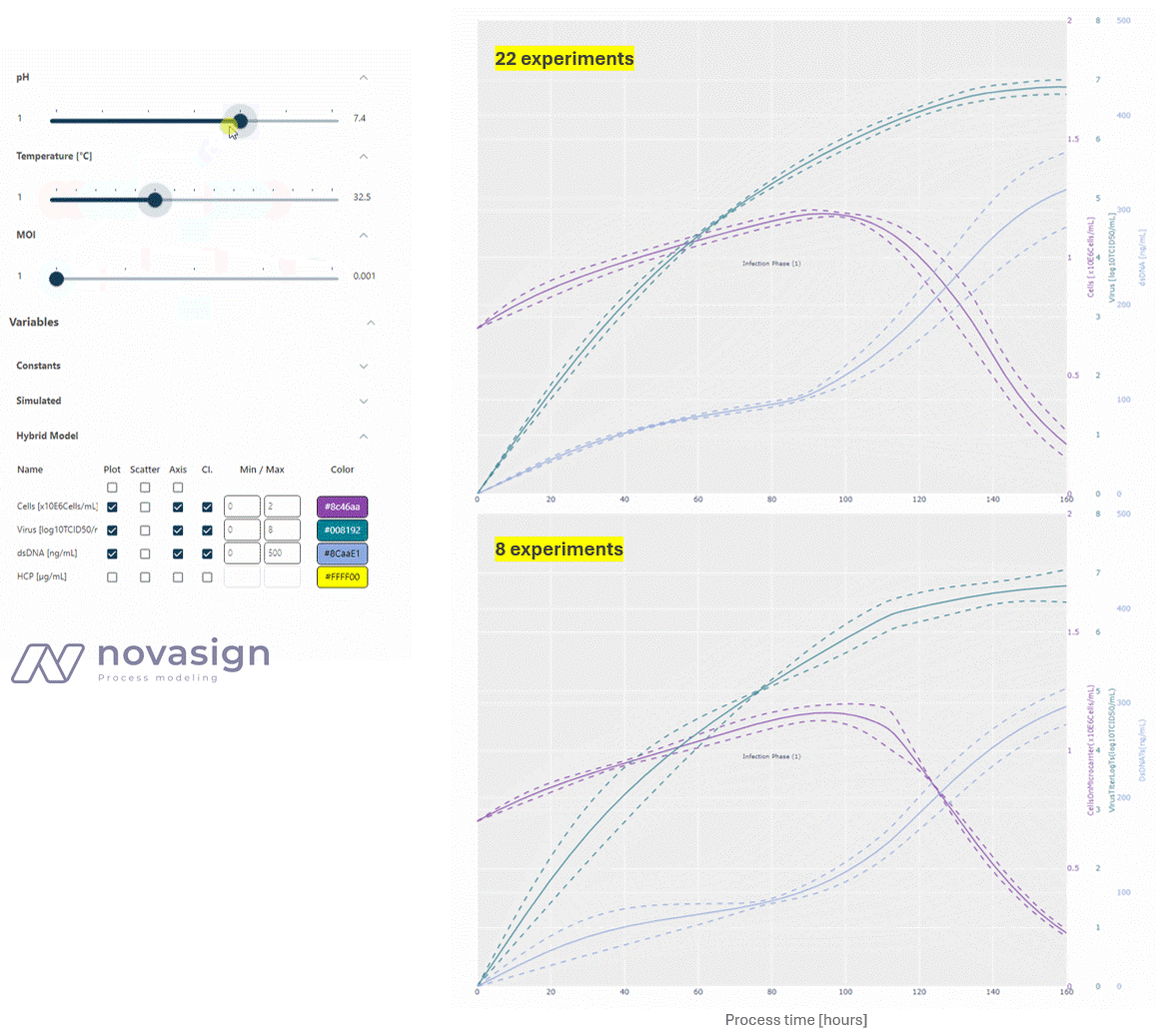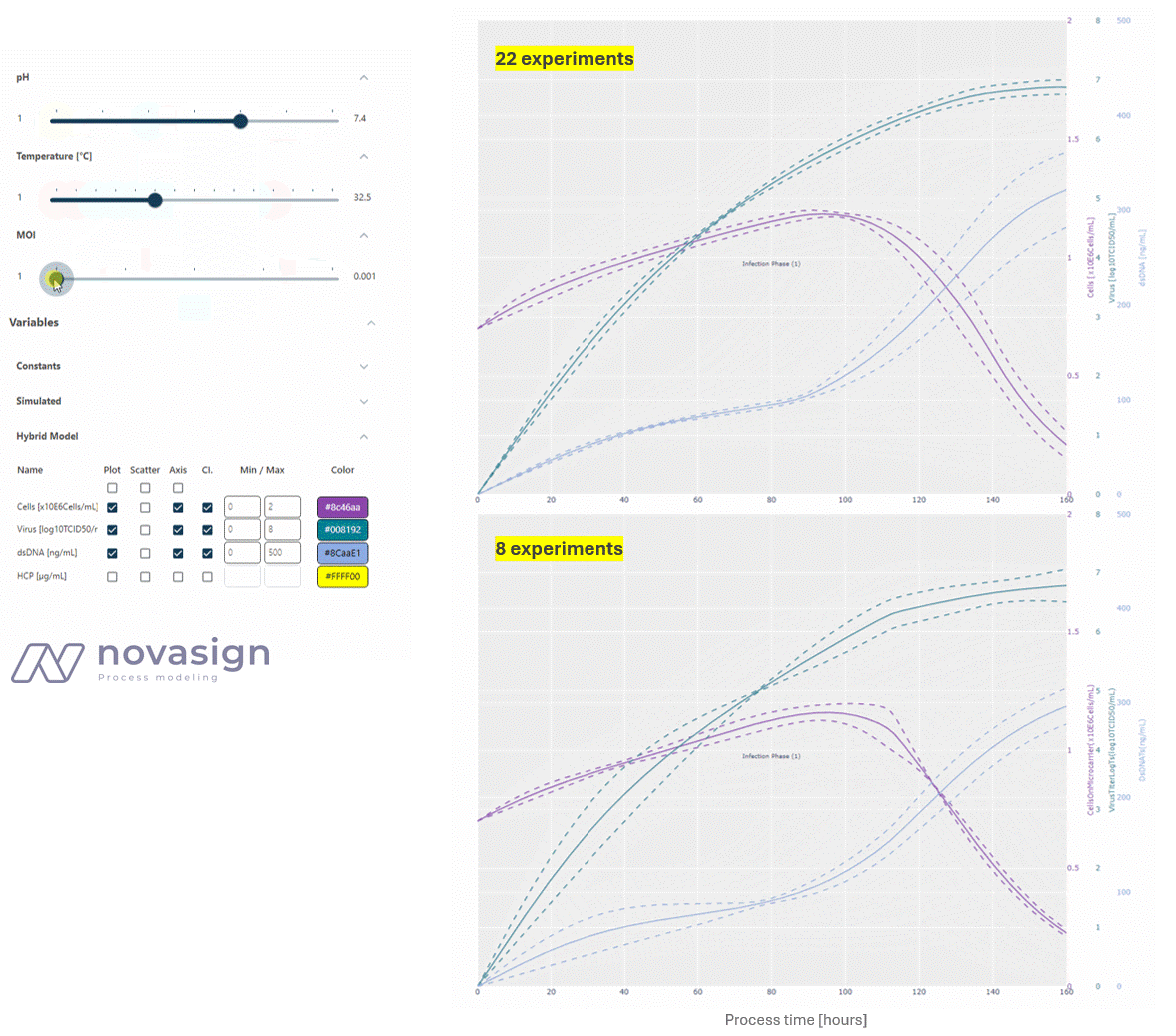
As the two simulations above show, the predictions of the model, whether based on 22 or 8 strategically placed experiments, are identical. This forms the basis for time-resolved process optimization, and answers questions such as ‘When should I stop my experiment?’ and ‘What is a good trade-off between product titers and impurity levels?’
Multi-objective Process Optimization
The model that we employed used a combination of mechanistic understanding of the bioreactor environment and machine learning. Using online and offline data, the model accurately predicted results for multiple outputs at once. The remaining 14 runs were then used for validating the model’s precision and robustness.
A key feature of the model is its time-resolved capability, allowing researchers to simulate and make strategic decisions such as:
- Optimizing Process Duration: At a low MOI, low temperature, and average pH, the process duration can be shortened by approximately 24 hours, while still increasing the titer and reducing dsDNA from 400 to 200 ng/ml compared to the initial platform process.
- Evaluating Integrated Processing: Interestingly, a key finding is that a higher MOI does not increase the virus titer, but it does lead to higher dsDNA impurities, adding stress to subsequent downstream units. This shows how adapting conditions in the cultivation process can simplify downstream operations.
State-of-the-art DoE studies typically focus only on endpoint determination and individual process units, limiting the ability to optimize the entire chain. This showcase demonstrates how holistic bioprocess optimization, where every decision considers its impact on the overall system, can ultimately lead to quantifiable improvements, which can be linked to better process economics.
Quantifying the Economic Impact
By understanding the concentration of the product and impurities at each time point, it is possible to assess the economic impact of these improvements. Achieving a 64% reduction in experimental runs translates into:
- €40k savings in material and operating costs for the experiments.
- 12 weeks reduction in laboratory work.
- 24 hours shorter process time per experiment.
Ready to Transform Your Process Development?
This showcase demonstrates how smart experimental design and hybrid modeling can redefine bioprocess development, enabling data-driven decisions with fewer experiments. Are you looking to optimize your processes while saving time and reducing costs? Contact us

